Abstract
Introduction Marginal zone lymphoma (MZL) is an indolent B-cell non-Hodgkin lymphoma that includes 3 subtypes: extranodal marginal zone lymphoma (EMZL) of the mucosa-associated lymphatic tissue (MALT), splenic MZL (SMZL), and nodal MZL (NMZL). Outside of case reports and small case series, the relevance of monoclonal protein (M-protein) in patients with MZL at diagnosis is largely unknown. Hence, we sought to evaluate the impact of M-protein at diagnosis on outcomes in patients with MZL.
Methods This multicenter, retrospective cohort study of MZL patients treated at 8 US medical centers included patients who were ≥18 years old, diagnosed with MZL from 2010-2020, and had information on M-protein at diagnosis. Patients who were on observation or received antibiotics only were excluded. Patients were grouped according to the presence or absence of M-protein detectable by serum protein electrophoresis or immunofixation. The primary endpoint was progression-free survival (PFS). Secondary endpoints were time from diagnosis to systemic therapy, cumulative incidence of relapse in stage 1 disease after local treatment (surgery or radiation therapy), and cumulative incidence of transformation in M-protein versus no M-protein groups. PFS was defined as the time from the start of first-line therapy until lymphoma relapse/progression or death from any cause, censoring at the last clinical assessment.
Results Among 501 eligible patients with newly diagnosed MZL, 152 (30%) had detectable M-protein at diagnosis and 349 (70%) did not. Among those with M-protein, 59% (n=90) had IgM, 29% (n=44) had IgG, 3% (n=4) had both IgM and IgG, and 9% (n=14) had non-IgM/non-IgG M-protein and light chains. Patients in the M-protein group had a higher median age (66 vs 61 years), more advanced stage disease (76% vs 53%), lower albumin (27% vs 15%), and were more likely to receive R-chemotherapy (39% vs 23%) compared to no M-protein group.
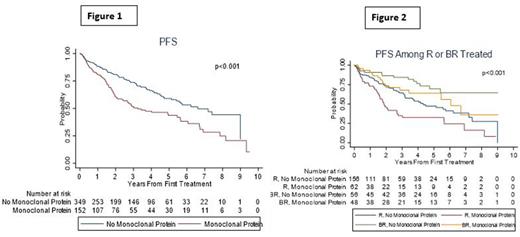
The median PFS was 3.2 years (95%CI=2.1-5.6) in the M-protein group compared to 6.6 years (95%CI=5.4-NR) in the no M-protein group (log-rank p<0.001; Figure 1) with a 3-year and 5-year PFS rates of 51% and 44% in the M-protein gp versus 74% and 59% in the no M-protein groups. In order to determine if the M-protein was independently associated with PFS, we performed Cox regression analysis in only those who received systemic therapy at diagnosis (n=377). After adjusting for factors associated with PFS in univariate analysis (age, MZL subtype, ECOG PS, albumin level, LDH>ULN, and first-line treatment regimen), M-protein remained associated with significantly inferior PFS (HR=1.80, 95%CI=1.24-2.62, p=0.002) in the multivariable analysis. There was no difference in PFS based on the type of M-protein at diagnosis (IgM vs IgG, p=0.88).
M-protein group treated with rituximab monotherapy had significantly shorter PFS compared to no M-protein group (1.9 years versus 4.3 years, p<0.001, Figure 2), however, there was no significant difference in PFS among patients treated with bendamustine and rituximab (BR, 6.1 years versus NR, p=0.14). In the EMZL subset (n=101) treated with local therapy (surgery or radiation), PFS did not significantly differ by presence/absence of M-protein at diagnosis (p=0.53).
There was no difference in the time from diagnosis to systemic therapy initiation between the M-protein and no M-protein groups with % of patients who started treatment at 1, 3, and 5 years being 89% vs 86%, 96% vs 94%, and 99% vs 98%, respectively (p=0.21). The cumulative incidence of relapse in stage 1 disease (n=144) among the recipients of local therapy was not significantly different between the M-protein and no M-protein groups (p=0.10). The cumulative incidence of transformation was significantly higher in the M-protein group compared to no M-protein group with 3-, 5-, and 10-year rate of transformation being 5.6% vs 0.6%, 8.9% vs 1.3%, and 14% vs 1.3%, respectively (p=0.001).
Conclusions In this study (to our knowledge, the largest to date) of the prognostic relevance of M-protein in MZL, we found that M-protein at diagnosis was associated with shorter PFS and a higher risk of histologic transformation. Because the PFS difference was not observed after BR, immunochemotherapy may be a preferred approach over rituximab monotherapy in this group and needs to be explored further. M-protein in stage 1 EMZL was not associated with shorter PFS after standard local therapy.
Disclosures
Epperla:Incyte: Speakers Bureau; Novartis: Honoraria; TG Therapeutics: Other: Ad Board; BeiGene: Other: Ad Board; Seattle Genetics: Other: Ad Board; Pharmacyclics: Other: Ad Board. Karmali:Kite: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau; BMS/Celgene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Other: Advisory Board; Karyopharm: Consultancy; Morphosys/Incyte: Consultancy, Other: Advisory Board, Speakers Bureau; Eusa: Consultancy; AstraZeneca: Other: Advisory Board, Speakers Bureau; Takeda: Research Funding; Genentech/Roche: Consultancy, Other: Advisory Board; Calithera: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; BeiGene: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau. Torka:Epizyme: Consultancy; Lilly USA: Consultancy; Genentech: Consultancy; ADC Therapeutics: Consultancy; TG Therapeutics: Consultancy; Targeted Oncology, Physician Education Review: Honoraria. Greenwell:Kyowa Kirin: Consultancy; Stemline: Consultancy. Sawalha:Epizyme: Consultancy; BeiGene: Research Funding; Celgene/BMS: Research Funding; TG Therapeutics: Research Funding. Christian:Triphase: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Membership on an entity's Board of Directors or advisory committees; Millennium: Research Funding; Seattle Genetics: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Celgene/Bristol-Myers Squibb: Research Funding; Acerta: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees. Grover:ADC: Other: Advisory Board; Genentech: Research Funding; Kite: Other: Advisory Board; Novartis: Consultancy; Tessa Therapeutics: Consultancy. Bartlett:Autolus, Bristol-Meyers Squibb, Celgene, Forty Seven, Janssen, Kite Pharma, Merck, Millennium, Pharmacyclics: Research Funding; Washington University School of Medicine: Current Employment; ADC Therapeutics, Roche/Genentech, Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Olszewski:Precision Bio: Research Funding; Adaptive Biotechnologies: Research Funding; Celldex: Research Funding; Acrotech Biopharma: Research Funding; Schrodinger: Consultancy; TG Therapeutics: Consultancy, Research Funding; Genmab: Consultancy, Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal